Our Better Safe Than Tumour campaign
Better Safe Than Tumour is our UK-wide campaign to reduce diagnosis times of brain tumours.
Our Better Safe Than Tumour campaign was built on all of the amazing groundwork done by our previous signs and symptoms awareness campaign – HeadSmart campaign.
While HeadSmart focused on spreading awareness of the common childhood signs and symptoms of a brain tumour, Better Safe Than Tumour also aimed to raise awareness of common brain tumour signs and symptoms in adults. We also gave the campaign a brand-new, attention-grabbing look and feel that gave it a closer link to The Brain Tumour Charity.
Although Better Safe Than Tumour was only launched in July 2022, we’ve already had an incredible impact:
- the campaign has reached more than 35,000,000 people (yes, that’s 35 MILLION!)
- includes 2,000,000 on Heart Radio
- and 5 million watching our video
- and 13.5 million people seeing the campaign on social media
- over 110,000 people have visited the Better Safe Than Tumour website
- our new symptom checker has been used over 38,000 times
- almost 75 people have downloaded our digital Better Safe Than Tumour packs to share
- nearly 50 people have ordered our physical Better Safe Than Tumour packs to share

Be a voice for change
By campaigning with The Brain Tumour Charity, you can help ensure the issues which affect the brain tumour community remain a political priority.
Our HeadSmart campaign led the way
Brain tumours (or brain tumors as they are known internationally) are the leading cause of childhood cancer deaths in the UK. Around 500 children and young people in the UK are diagnosed each year. Diagnosis times of childhood brain tumours are longer in the UK than in many other countries.
Based on scientific research into the causes of these delays, funded by The Brain Tumour Charity, the HeadSmart campaign aims to reduce brain tumour and brain cancer diagnosis times to four weeks or less.
HeadSmart helped raise national awareness of the common signs and symptoms of a brain tumour in children and young people by equipping parents, the public and healthcare professionals with information they need. This was achieved through the distribution of symptom cards and posters to raise awareness of symptoms, and the promotion of a clinical guideline and training module for health professionals.
The impact of our HeadSmart campaign
Before the launch of HeadSmart, average diagnosis times for children with brain tumours in the UK was 13 weeks.
After publication of the guidelines for healthcare professionals in 2011, this was reduced to 9.1 weeks.
Following the public launch this was reduced to 7.5 weeks in 2012, 6.9 weeks in 2013, and most recently 6.5 weeks. With the launch of our Better Safe Than Tumour campaign, we want to reduce diagnosis times to four weeks or less to be on a par with, or better than, the rest of the world.
Our HeadSmart partners
HeadSmart was developed in collaboration with the Children’s Brain Tumour Research Centre at The University of Nottingham and the Royal College of Paediatrics and Child Health.
It was led and funded by The Brain Tumour Charity and received additional funding during the development and launch phase from the Healthcare Foundation.
HeadSmart was supported by a wide range of other organisations including the Royal College of GPs, the Royal College of Radiologists, the College of Emergency Medicine and the Children’s Cancer and Leukaemia Group.
It was shortlisted for a British Medical Journal Improving Health Award.
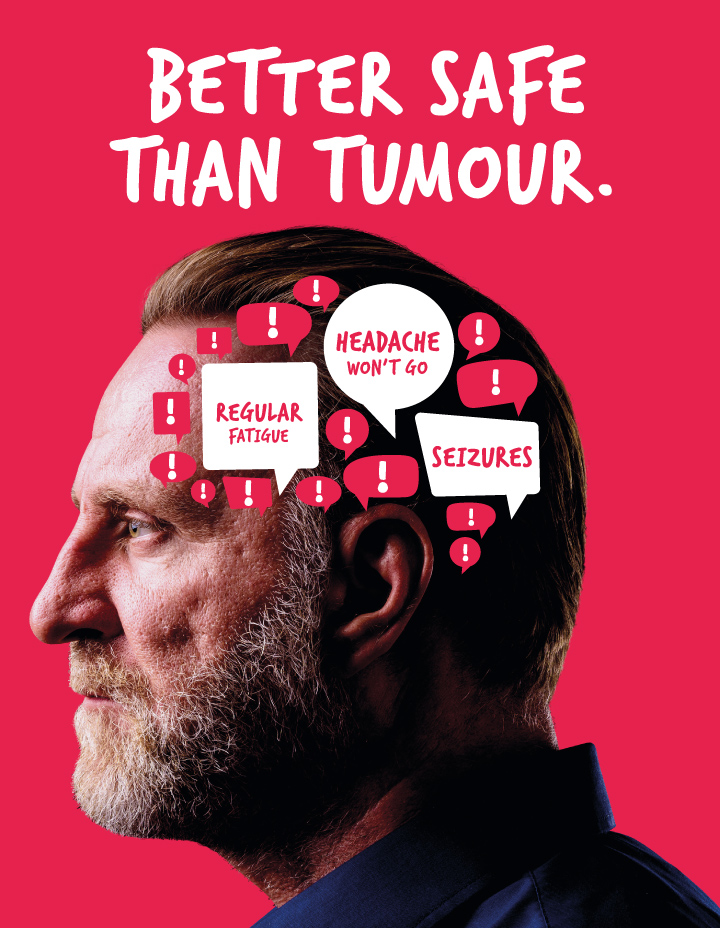
Know the Signs and Symptoms
Although brain tumours are rare, if you or a loved one are experiencing two or more of the signs and symptoms it’s important that you speak to your doctor to rule out a brain tumour.

Watch our Better Safe Than Tumour campaign video
Sam, named after Samantha Dickson, helped raise awareness of the common signs and symptoms of a brain tumour in children.
